Abstract
Background: AL amyloidosis is a rare disorder caused by deposition of insoluble amyloid fibrils composed of misfolded light chains in vital organs, such as the heart or kidney, resulting in serious and life-threatening organ dysfunction. Cardiac involvement is the primary determinant of clinical outcomes for AL amyloidosis, with survival expectations decreasing with increasing cardiac stage. Until recently, an approved treatment option for AL amyloidosis was not available, and the standard treatment approach utilized multiple myeloma therapies. After receiving accelerated approval, subcutaneous DARA (DARA SC) in combination with VCd became the first and only approved treatment for newly diagnosed AL amyloidosis. In the phase 3 ANDROMEDA study, hematologic complete response rate was consistently higher with D-VCd versus VCd in patients with newly diagnosed AL amyloidosis, regardless of baseline cardiac stage (Kastritis E, et al. N Engl J Med. 2021;385[1]:46-58). However, cardiac involvement provides challenges in the attribution of cardiac events to underlying disease and/or treatments. The ongoing phase 2 AQUARIUS (AMY2009) study will characterize the cardiac safety of different D-VCd treatment regimens in patients with newly diagnosed AL amyloidosis with cardiac involvement to identify potential mitigation strategies for cardiac toxicity, as well as characterize the pharmacokinetics of D-VCd among racial and ethnic minorities.
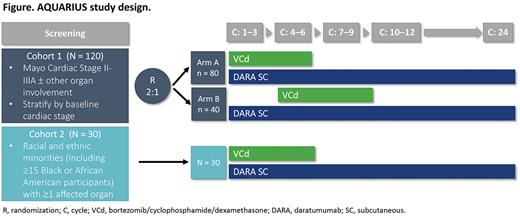
Study Design and Methods: This ongoing multicenter, multicohort, open-label phase 2 study will enroll approximately 150 patients aged ≥18 years with newly diagnosed systemic AL amyloidosis and measurable disease from 45 sites in 15 countries. Patients in Cohort 1 (n = ~120) will have cardiac involvement (AL amyloidosis Mayo Cardiac Stage II and Stage IIIa) with or without other organ involvement. Patients in Cohort 2 (n = ~30) will be of racial or ethnic minority (including ≥15 Black or African American participants) and have ≥1 organ impacted by systemic AL amyloidosis according to consensus guidelines.
Patients in Cohort 1 will be stratified by baseline cardiac stage (Stages II and IIIa) and randomly assigned (2:1) to DARA SC plus immediate VCd (Arm A) or DARA SC plus deferred VCd (Arm B; Figure). In both arms, DARA SC (1,800 mg DARA co-formulated with recombinant human hyaluronidase PH20 [rHuPH20; 2,000 U/mL; ENHANZE® drug delivery technology, Halozyme, Inc.]) will be administered weekly (Day 1) during Cycles 1-2, every 2 weeks (Days 1 and 15) during Cycles 3-6, and every 4 weeks (Day 1 of each cycle) thereafter until a maximum of 24 cycles or start of subsequent therapy. In Arm A, starting at Cycle 1 Day 1, VCd will be administered weekly (Days 1, 8, 15, and 22) in every 28-day cycle for a maximum of 6 cycles (Cycles 1-6). In Arm B, starting at Cycle 4 Day 1, VCd will be administered weekly (Days 1, 8, 15, and 22) in every 28-day cycle for a maximum of 6 cycles (Cycles 4-9). Patients in Cohort 2 will receive DARA SC plus immediate VCd.
The first primary endpoint is incidence of any toxicity grade cardiac events for the different D-VCd treatment regimens (Arm A: DARA SC + immediate VCd; Arm B: DARA SC + deferred VCd). Cardiac safety assessments will include TroponinT, high sensitivity (HS) TroponinT, and N-terminal pro-brain natriuretic peptide (NT-proBNP); transthoracic echocardiogram; electrocardiogram; New York Heart Association (NYHA) assessment; functional 6-minute walk test; and a focus on clinical cardiac signs and symptoms at baseline and their potential changes during the study. The second primary endpoint is trough serum concentration (Ctrough) at the end of weekly dosing (Cycle 3 Day 1 predose). Efficacy evaluations will include assessment of hematologic response and organ response. Data will be summarized using descriptive statistics. The first patient was enrolled on March 17, 2022. The ClinicalTrials.gov identifier is NCT05250973.
Disclosures
Sanchorawala:Proclara, Caelum, Abbvie, Janssen, Regeneron, Protego, Pharmatrace, Telix, Prothena: Consultancy; Celgene, Millennium-Takeda, Janssen, Prothena, Sorrento, Karyopharm, Oncopeptide, Caelum, Alexion, Pfizer, Janssen, Attralus, Proclara, Caelum, Abbvie, Janssen, Regeneron, Protego, Pharmatrace, Telix, Prothena: Consultancy, Research Funding, Speakers Bureau. Rosenzweig:Bristol Myers Squibb: Speakers Bureau; Janssen: Research Funding, Speakers Bureau; Pfizer: Research Funding; Akcea: Speakers Bureau; Prothena: Honoraria; Janssen, BMS, Akcea: Research Funding, Speakers Bureau. Pericone:Janssen Research & Development: Current Employment. Vasey:Janssen Research and Development: Current Employment, Current equity holder in publicly-traded company. Qin:Janssen: Current Employment. Kotoulek:Janssen Research and Development: Current Employment. Vandael:Janssen Research & Development: Current Employment. Duran:Janssen Research and Development: Current Employment. Khaled:Janssen Research & Development, LLC: Current Employment. Tran:Janssen Research & Development, LLC: Current Employment, Current equity holder in publicly-traded company. Kastritis:Takeda: Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; GSK: Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Genesis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal